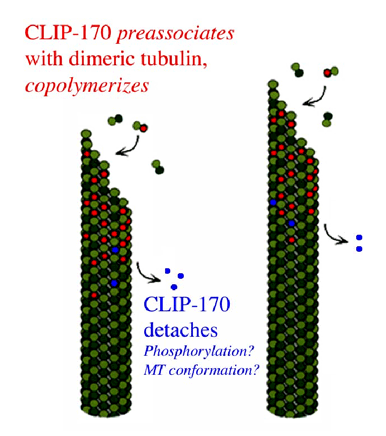
α-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons - ScienceDirect

Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition | PNAS
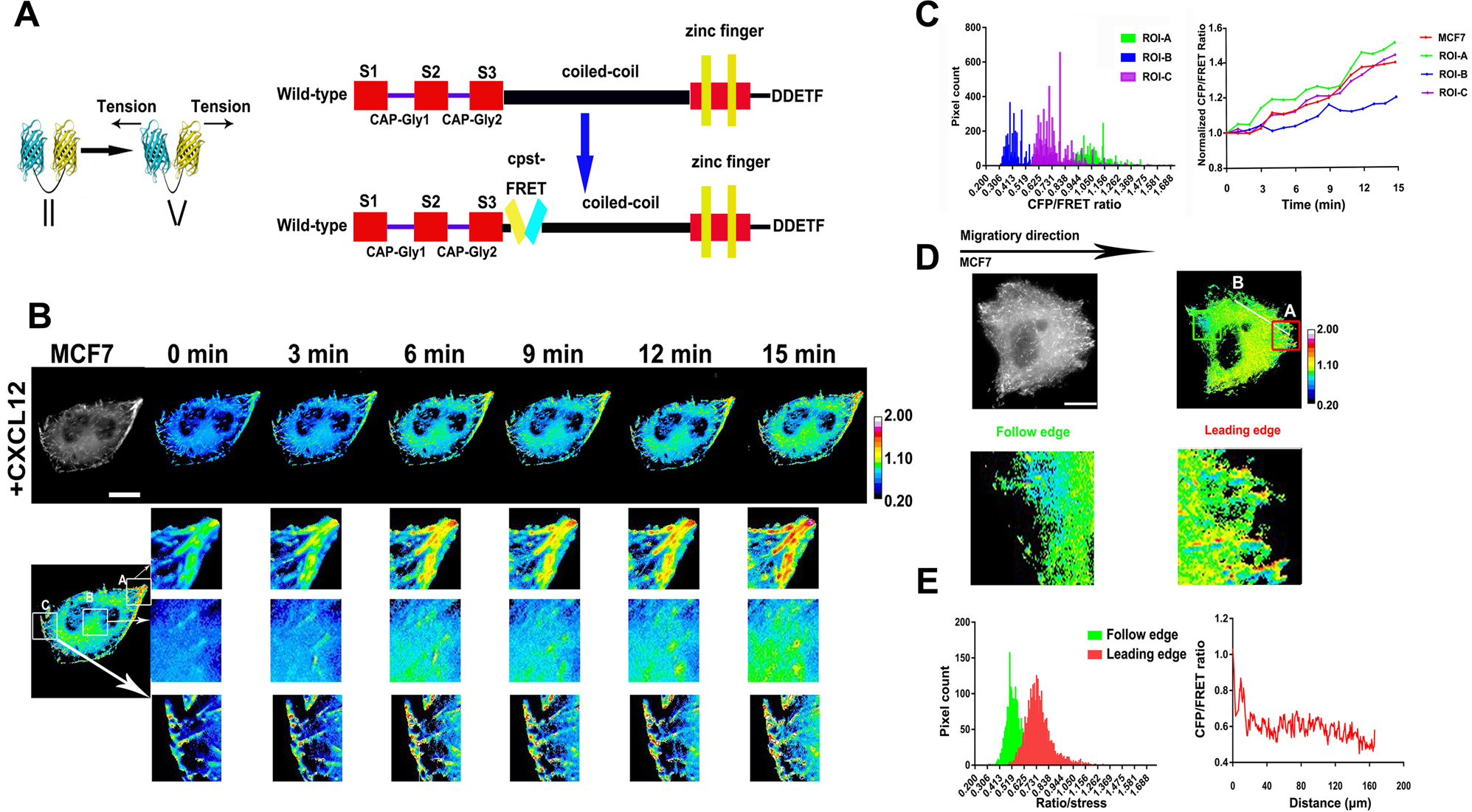
Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration | Cell Death & Disease

Phosphorylation of CLIP‐170 by Plk1 and CK2 promotes timely formation of kinetochore–microtubule attachments | The EMBO Journal

Drosophila CLIP-190 and mammalian CLIP-170 display reduced microtubule plus end association in the nervous system | Molecular Biology of the Cell

Interactions between CLIP-170, Tubulin, and Microtubules: Implications for the Mechanism of CLIP-170 Plus-End Tracking Behavior | Molecular Biology of the Cell
The CLIP-170 N-terminal domain binds directly to both F-actin and microtubules in a mutually exclusive manner
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular

Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres | Open Biology
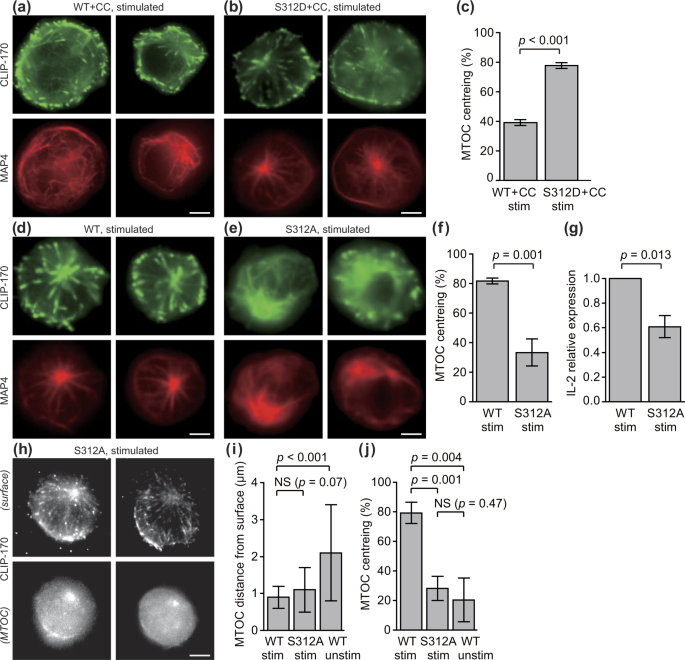
CLIP-170 is essential for MTOC repositioning during T cell activation by regulating dynein localisation on the cell surface | Scientific Reports
Selective visualization of growing MT ends with CLIP170. CHO cells were... | Download Scientific Diagram

Quelle fonction pour la CLIP-170? : recherche de partenaires et nouveaux outils d'investigation | Semantic Scholar

Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. - Abstract - Europe PMC
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE

Microtubule binding proteins CLIP-170, EB1, and p150Glued form distinct plus-end complexes - ScienceDirect
Cdc2-mediated Phosphorylation of CLIP-170 Is Essential for Its Inhibition of Centrosome Reduplication*